Search for products
Shirt with cooling collar and cooling pockets
Brief Description
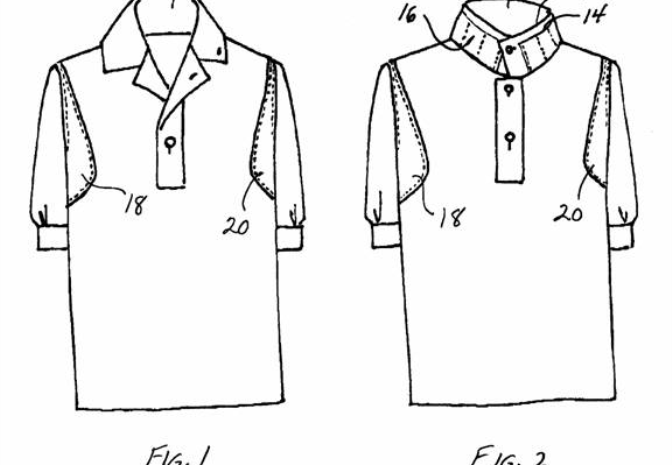
This shirt includes a collar with inner and outer layers forming ice pockets spaced around the collar. The pockets are for ice to cool the main blood vessels and spinal nerves in the neck of the wearer. In use, the collar is either in an upstanding closed position around the wearer's neck in a turtleneck fashion, or in a spread-open position if the closed collar is too warm or too cold. The shirt also includes ice pockets behind armpit locations where arteries are close to the skin. The shirt enhances the transfer of heat from the wearer to mel
Request More Information Sign Up for Product AlertsProduct Details
This shirt includes a collar with inner and outer layers forming ice pockets spaced around the collar. The pockets are for ice to cool the main blood vessels and spinal nerves in the neck of the wearer. In use, the collar is either in an upstanding closed position around the wearer's neck in a turtleneck fashion, or in a spread-open position if the closed collar is too warm or too cold. The shirt also includes ice pockets behind armpit locations where arteries are close to the skin. The shirt enhances the transfer of heat from the wearer to melt ice in the ice pockets, to warm the melted water, and to evaporate the water.
There are three heat transfer processes occurring in the system of this invention:
1) Melting; Ice enclosed in a moisture-permeable material absorbs heat from adjacent skin of the wearer. Melting ice makes the material wet.
2) Heating; Heat continues to transfer from the wearer to the wet material until the material reaches body temperature.
3) Evaporation; Water evaporates from the material. Evaporation is a cooling process in which the evaporating medium (water) absorbs heat from a heat source (skin).
All of these processes are cooling processes; they absorb heat from the object being cooled. Evaporation is by far the most effective of these cooling processes because the "heat of vaporization" of water is nearly seven times its "heat of fusion". As an example: An ounce of ice at 32° F. absorbs 9 BTU from a heat source (skin) to become water at 32° F. That ounce of water then absorbs 4 BTU from the skin to reach 98° F. body temperature. Then, that ounce of water as it evaporates absorbs 61 BTU from the skin.
Product Selling Benefits
Keep focus on the job in hot day. Prevents heat exhaustion in hot environments. Prevents falling asleep while driving a car.
Products Specs
| Seller Objective: | Looking to license Intellectual Property / Patent rights |
| Patent / IP Status: | Utility patent issued |
| Inventory in Stock: | Inventory has never been ordered |
| Number of Units in Stock: | None |
| Suggested Retail Price: | N/A |
| Wholesale Price: | N/A |
| Estimated Cost/Unit: | N/A |
| Minimum Order/Units: | N/A |
| Prior Sales Activity: | None sold to date |
Print Page